Drug development for fibrotic diseases
Fibrosis is a detrimental condition that appears in numerous medical disorders and is estimated to be involved in ~45% of all deaths in the developed world. It results from dysregulated repair of damaged tissues and involves excess deposition and crosslinking of extracellular matrix (ECM) proteins, resulting in tissue stiffening. Normally, tissue repair includes two major steps: replacement of the injured or missing cells by cells of the same type, and deposition and organization of new ECM at the injured site.
In fibrosis, however, deregulated signaling prevents termination of the repair process, and as a result ECM is continuously deposited at the expense of resident tissue cells, leading to further tissue stiffening, thus perpetuating the feed-forward loop that ultimately results in disrupted architecture of the tissue which prevents proper organ function.
Based on our lab’s previous research and expertise in studying the ECM assembly machinery, we aim to develop new therapeutic approaches for fibrotic pathologies using various disease models.
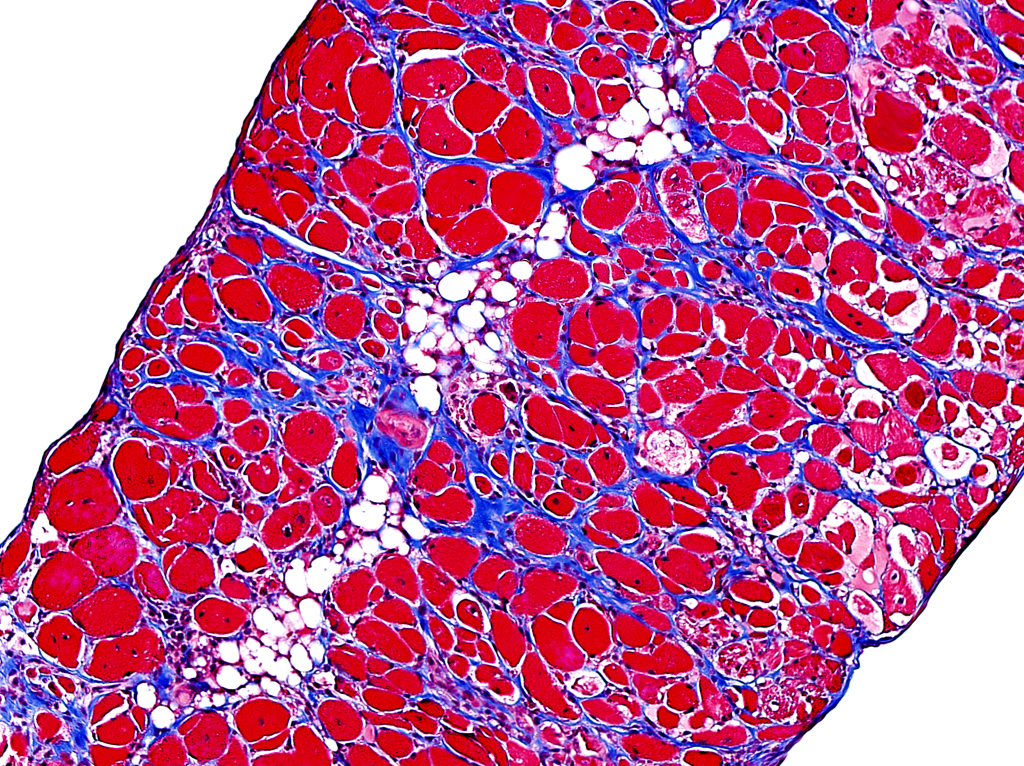
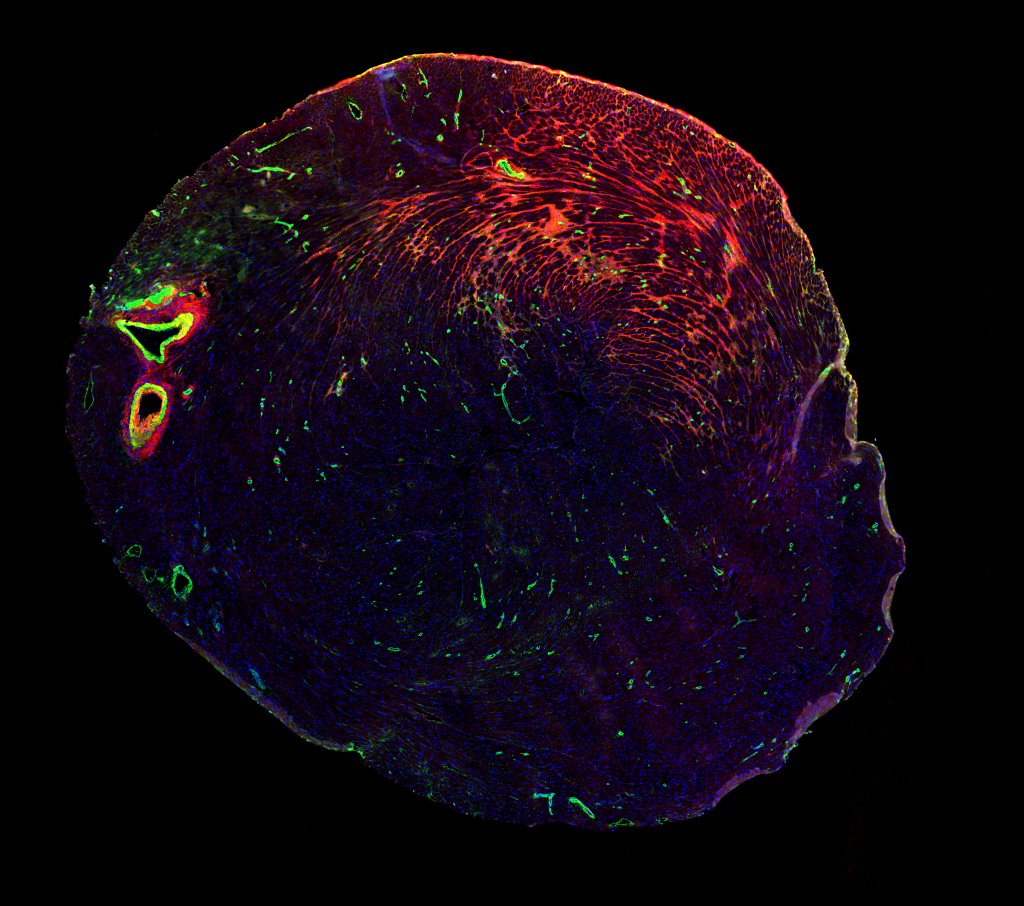
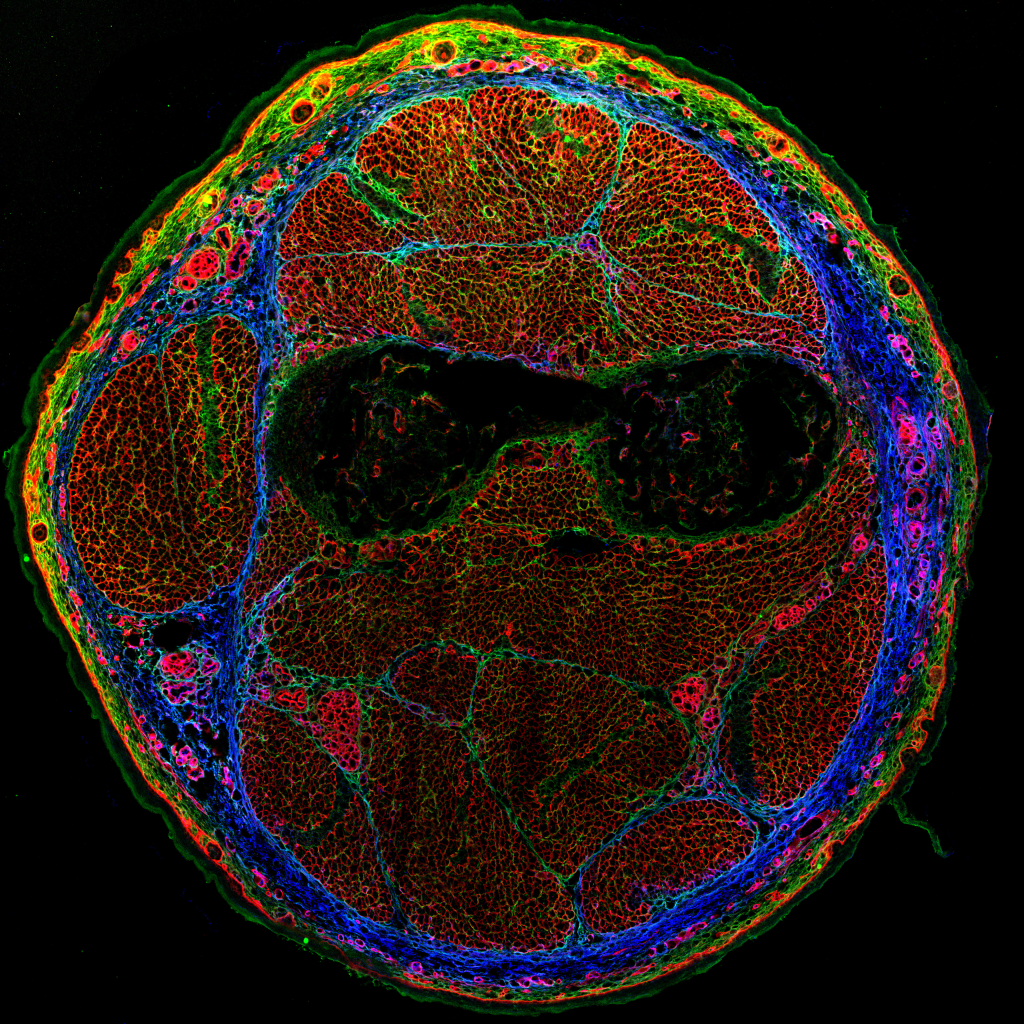
Lox and ECM Remodeling in the cardiovascular system
The cardiovascular system acts as the lifeline of our bodies, circulating the blood for the transport of nutrients to maintain the body’s homeostasis. This system is able to sense and adapt to various stressful conditions, such as hypertension, through a process referred to as cardiovascular remodeling, an important mechanism to preserves cardiac output and vascular integrity. Failure of the cardiovascular system to respond to these harmful conditions can lead to multiple pathologies such as cardiac overload or aneurysms.
Although these remodeling events have been well documented, how their underlying intra- and extracellular machineries interconnect, what triggers them and what cells are responsible for their induction remains unclear. With an aim at addressing these questions, we specifically target Lysyl oxidase (Lox), a key ECM modifying enzyme highly expressed in smooth muscle cells and fibroblasts. Using our conditional knockout mice, we delete Lox in multiple cellular lineages to follow its specific activities and monitor broad ECM remodeling processes in the cardiovascular system.
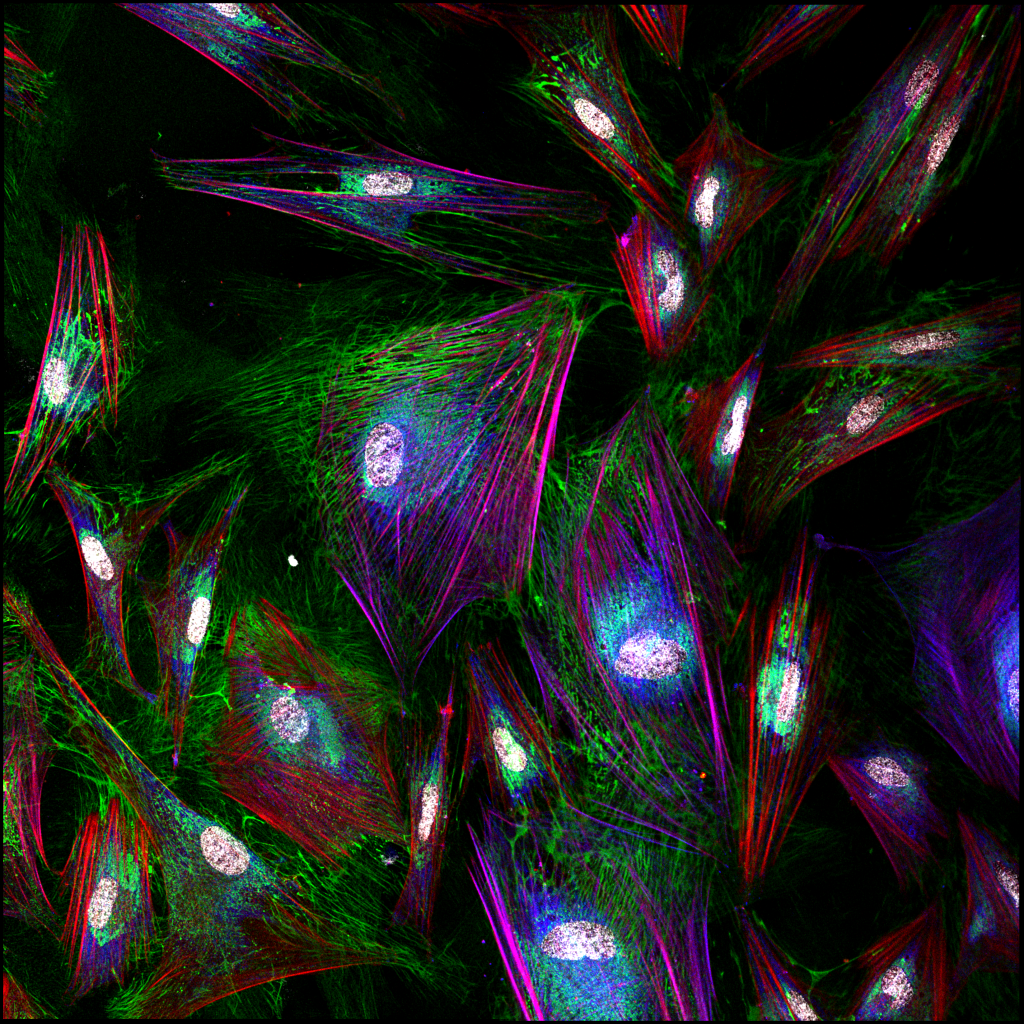
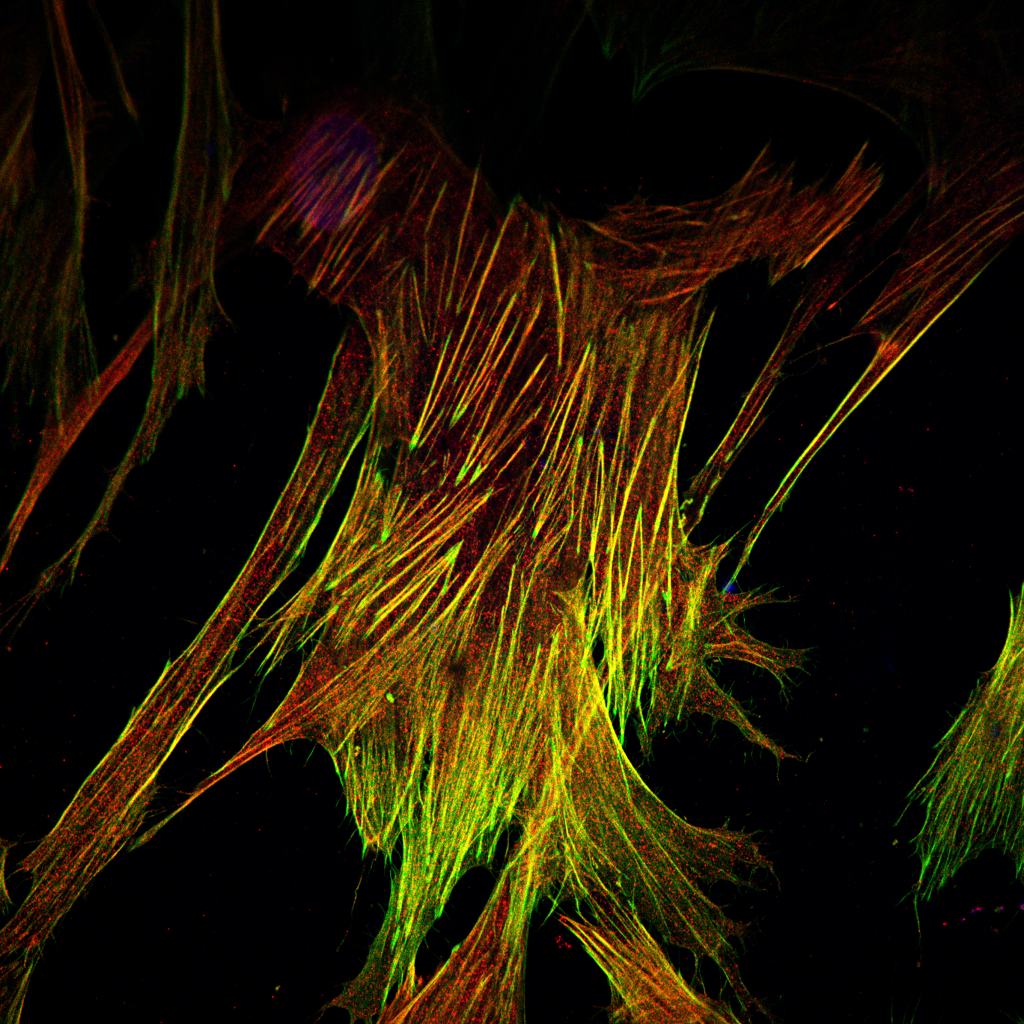
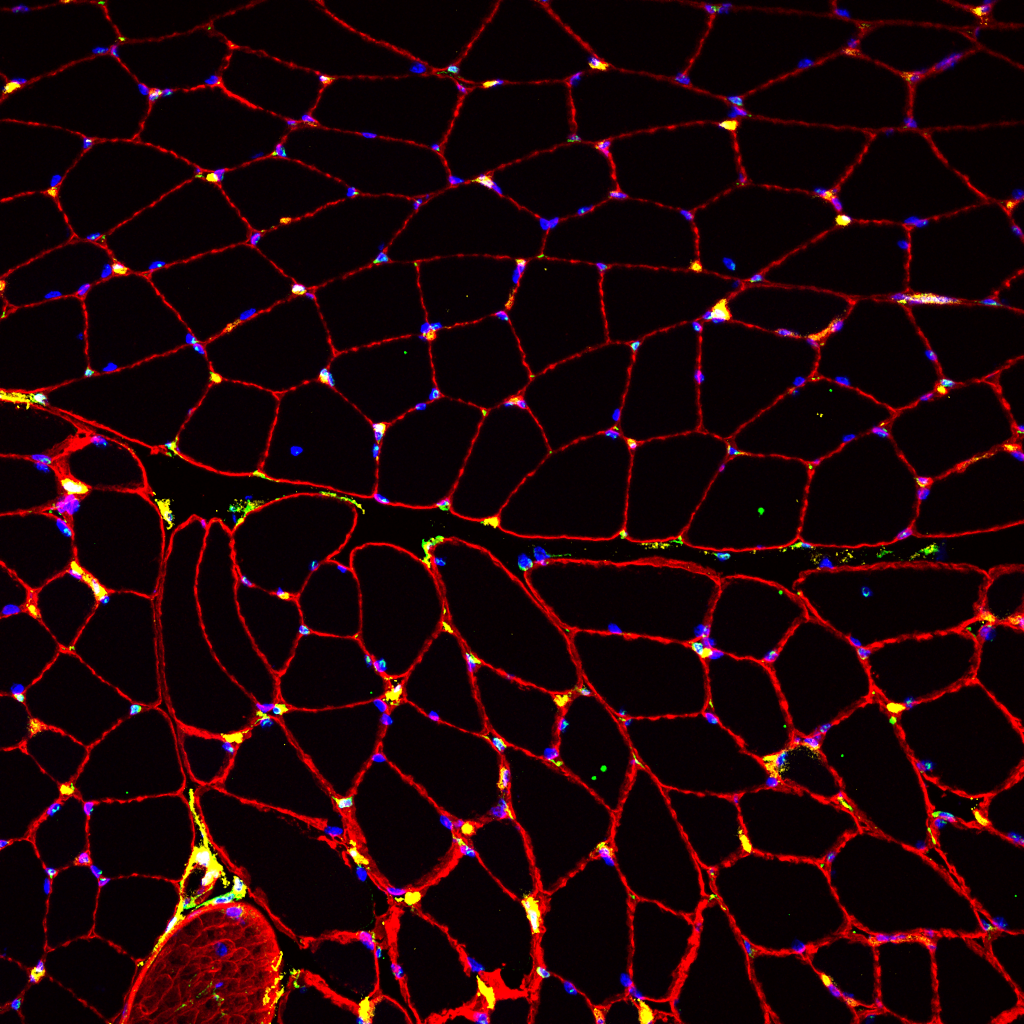
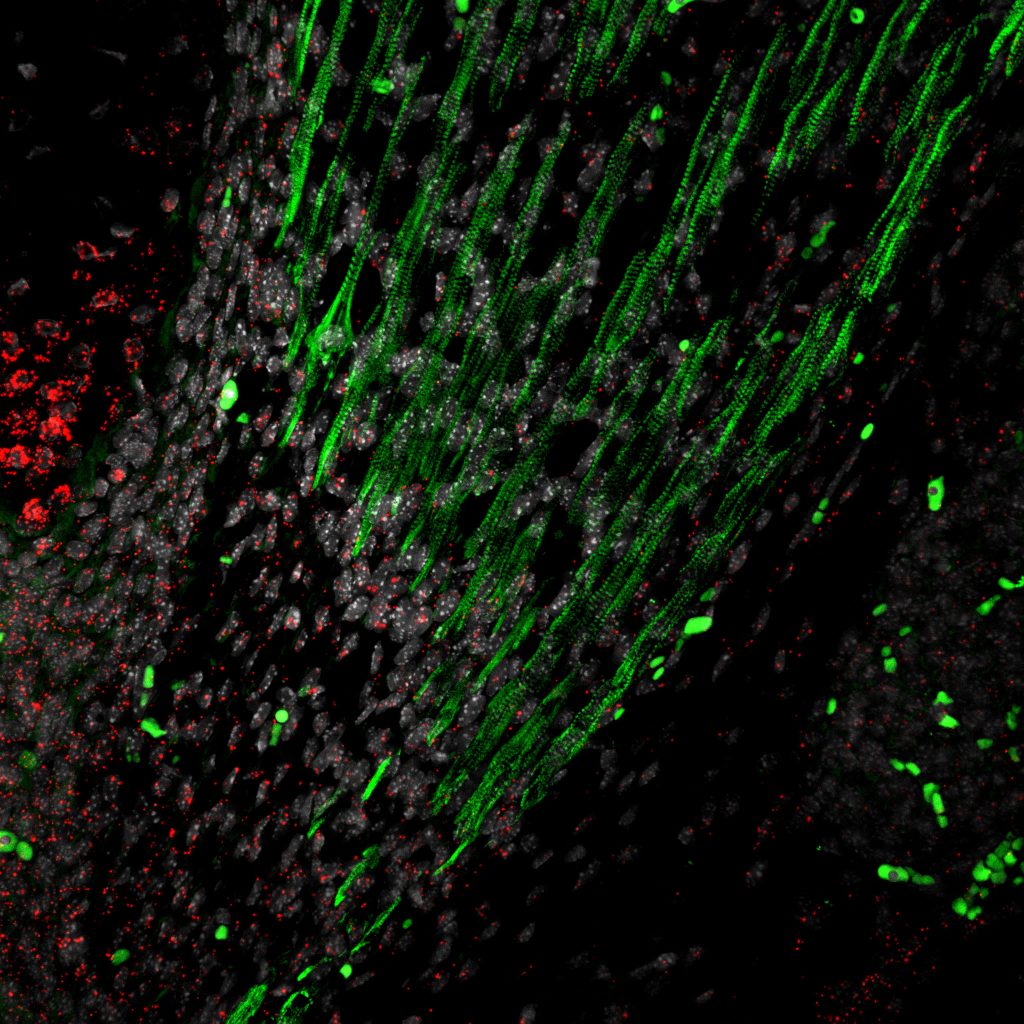
Fibroblast heterogeneity in embryonic muscle development
The musculoskeletal system is a multicomponent unit composed of three basic tissues: skeleton, for structure; muscles, the main force-producing tissue providing contractile forces that mobilize the skeleton; and tendons, for connecting and transmitting forces between the muscles and the skeleton. However, another major yet relatively overlooked component of this system is the muscle connective tissue (MCT) which surrounds, protects, and interconnects these primary components and is involved in force transmission. The MCT is primarily composed of extracellular matrix (ECM) and its resident producing cells, the fibroblasts.
Fibroblasts are the most common cell type found in our bodies’ connective tissues, where they play key roles in virtually all processes ranging from early stages of embryonic development through adulthood to the aging tissue. While fibroblasts were considered for many years as cells with relatively little divergent characteristics, recent observations have demonstrated that fibroblasts are heterogeneous groups of cells with distinct transcriptomes, suggesting that each of these subpopulations has unique roles. Yet how these fibroblasts diversify and what are the specific roles the various subpopulations play, remain largely unknown.
Towards answering these questions, we take advantage of the well characterized process of embryonic muscle development, combining computational and experimental approaches.
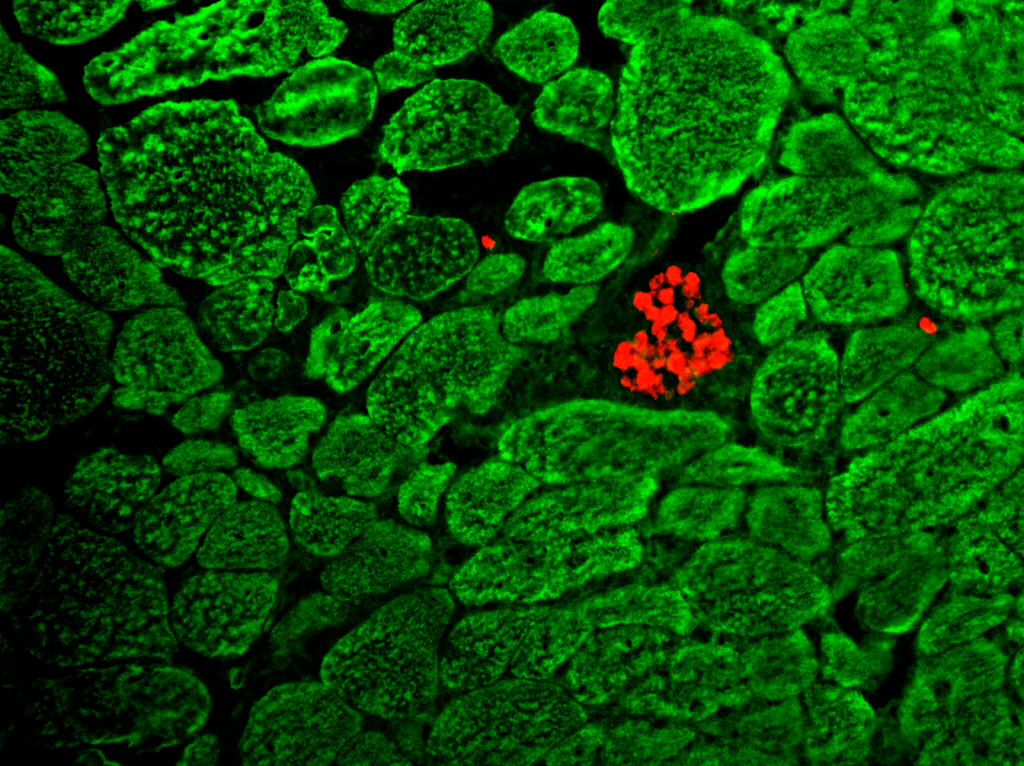
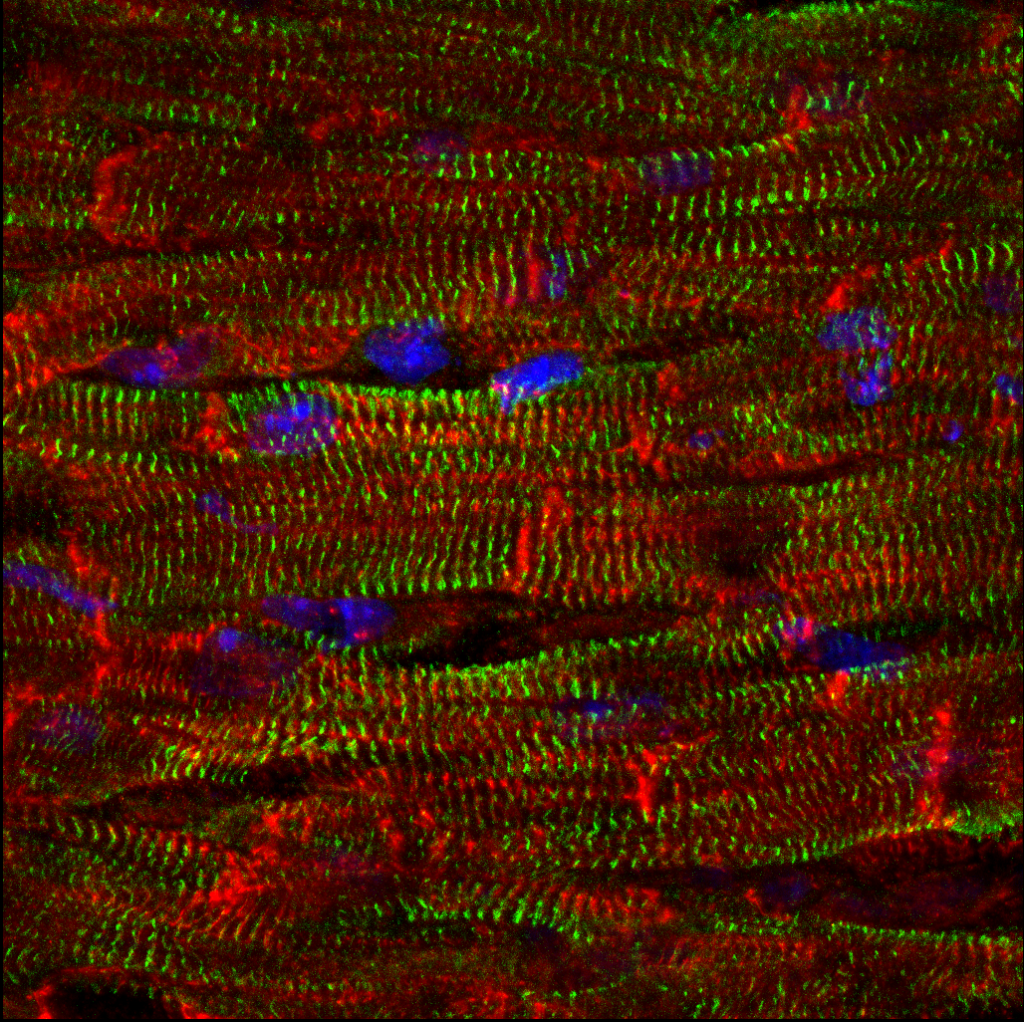
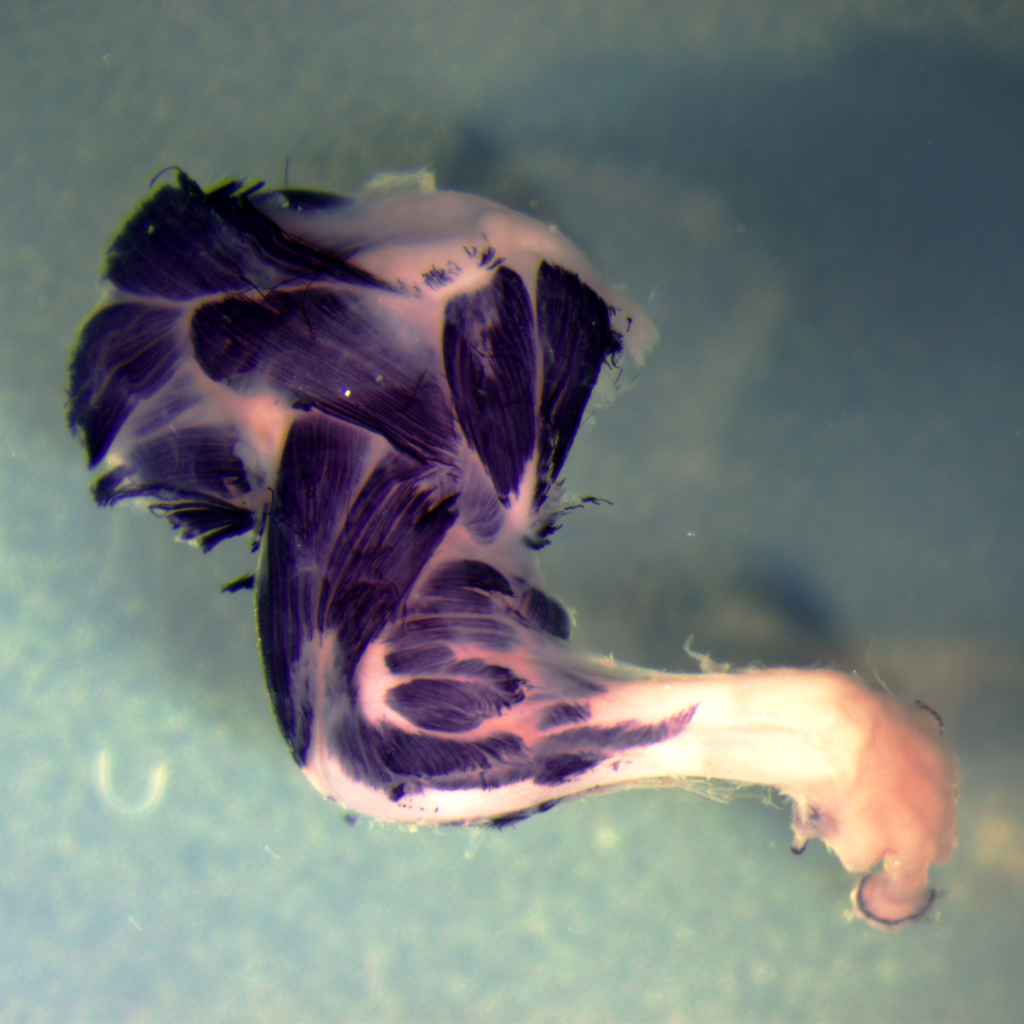
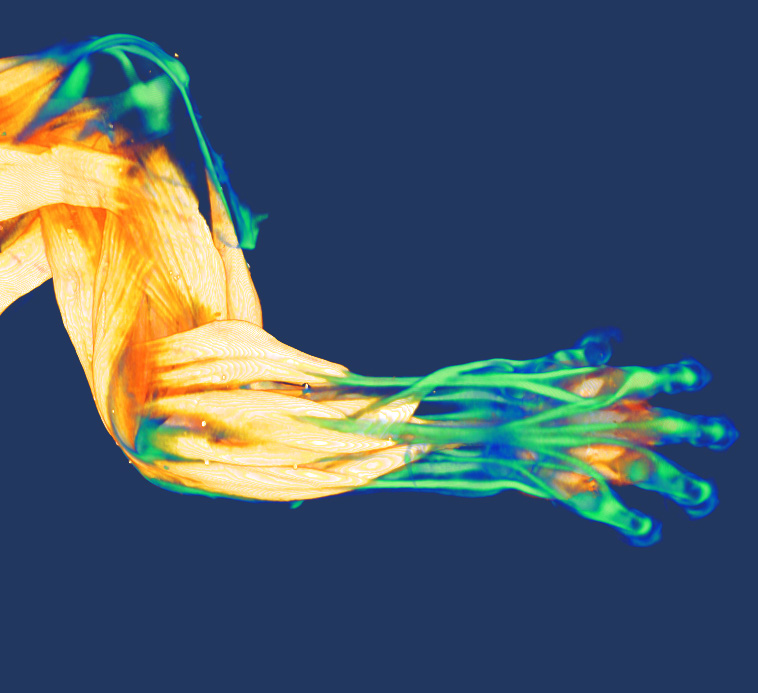
Muscle-Tendon Junction development
For muscles to function, myofibers have to stretch and anchor at the myotendinous junction (MTJ), a region rich in extracellular matrix (ECM), and a site where cells and tissues of distinct embryonic lineages interact and integrate. Although being critical for muscle functioning, our understanding of the mechanisms that underlie MTJ development and maintenance are still relatively unclear.
In our lab, we examine the mechanisms underlying MTJ formation at both cellular and molecular levels. Our work has revealed a novel process where cells of embryonic lineages foreign to the muscle are recruited into the myofibers along the MTJ. This process is essential for normal MTJ development ensuring proper localization of key ECM modifying enzymes along these junctions. However the exact mechanism facilitating this process, and the role of hybrid fiber formation in other contexts remain to be further explored.